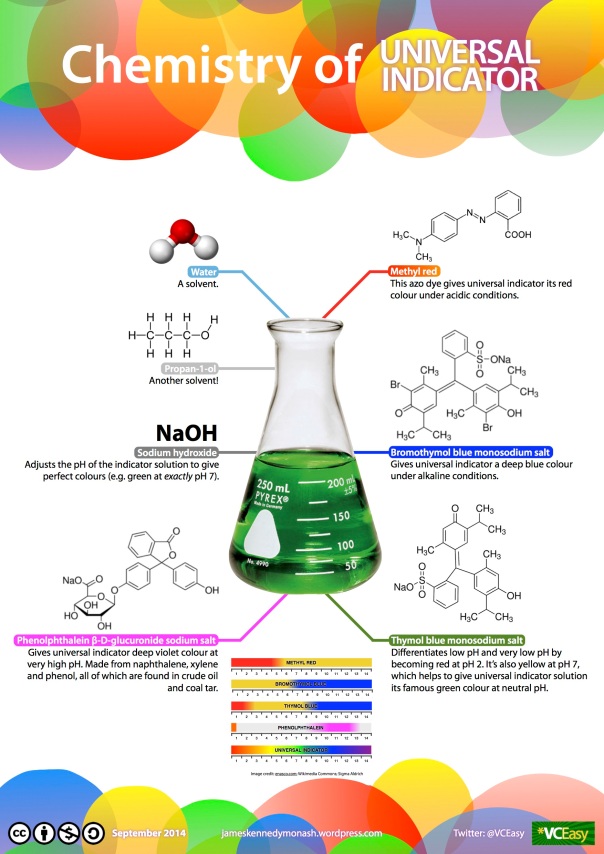
By definition, an indicator is a substance that changes colour in different pH environments. Universal indicator is a brown-coloured solution—containing a mixture of indicators—that can be added to any substance to determine its pH. Like all indicators, universal indicator changes colour in different pH environments. At low pH, it appears red, and at high pH, it appears blue or violet. At neutral pH, it appears green. Universal indicator can form a continuous spectrum of colours that give an approximate reading of the concentration of protons in a sample.
Water and propan-1-ol are used as solvents. They are both polar and dissolve all the other ingredients in the solution. Sodium hydroxide (NaOH) is an alkaline solution that adjusts the pH of the universal indicator to ensure that each colour is shown at the correct pH value. It is necessary to add NaOH to the universal indicator because some of the indicator compounds (e.g. methyl red) are acidic themselves, which would affect the colour of the other indicators present. NaOH is added to neutralise the solution.
Methyl red is red at pH <5 and yellow at pH >5. It provides orange and red hues to the universal indicator solution at low pH. The end point of an indicator compound is defined as the pH at which it changes colour. The end point of methyl red, therefore, is somewhere around pH 5.
Bromothymol blue is blue at pH >6 and yellow at pH <6. It gives blue and indigo hues at high pH. Its end point is therefore around pH 6.
Thymol blue has two end points: it is red below pH <2, blue at pH >8 and yellow in the middle. Thymol blue allows universal indicator to differentiate low and very low pH by providing another red hue below pH 2. Thymol blue is yellow at pH 7, which, when combined with bromothymol blue (which is blue at pH 7), give a green colour.
Finally, phenolphthalein gives universal indicator a deep violet colour at very high pH.
This 2-miunte BBC video is a great introduction to universal indicator:
I love chem. You put a thing that is a non poison with another non poison and you get the murder clue to a mystery novel.
LikeLike
How about the formation of esters? Two noxious ingredients combined with a corrosive acid catalyst can give you a fragrant, edible compound that smells of pineapple…!
LikeLike
pineapple?
LikeLike
Thanks for the video.
LikeLike
Reblogged this on not only chemistry.
LikeLike
Graphics not only present the theme behind the data connectivity but they also save a lot of time and make things easy to understand. Find a wide range of info graphic design and submit free infographic at LoveInfographics. Info@loveinfographics.com
LikeLike
Graphics not only present the theme behind the data connectivity but they also save a lot of time and make things easy to understand. Find a wide range of info graphic design and submit free infographic at LoveInfographics. http://www.loveinfographics.com/
LikeLike
I am searching the preparation procedure of Universal indicator from these components which I found in many websites on internet. But in this site also I am disapponited after not finding the extact amount of each component amd method of preparation of ultimate universal indicator which can be used finally for testing different solutions. If you have any problem in sharing it in internet can you send it to me via e-mail attachment? My email id is subhdatta@gmail.com.
LikeLike
Hello!
If you were sent the exact method of preparing indicators with quantities, then send them to me please e-mail
LikeLike
thank you for share this posters they are very cool But these images can not be used because it would be difficult translation
And if the are pdf
Is easily translated
LikeLike
thank you ,they are very cool
But these images can not be used because it would be difficult translation
And if
pdf
Is easily translated
LikeLike
Sir,
Thanks for valuable information. I have a little query. I would like to ask, in which colors the indicators are available? I have seen them in orange color. Are they available in others too or just orange?
Thanks in advance.
LikeLike
Universal indicator tends to be orange but changes colour when you add it to an acid or a base
LikeLike
Thank for the great information about universal indicator. It helped me a lot
LikeLike
Hi, I had this question : Why can universal indicator not be used with salts in a solid form?
LikeLike
because it’s a mixture of weak acids… and weak acids donate protons in aqueous solution. Those protons (H+ ions) can’t dissociate in solid form!
LikeLike
Thank you so much!
LikeLike
Hello James, Thank you so much for studying chemistry….further! I absolutely love, love, love your website. I make references from it for my A level chemistry students! Keep it up!!
LikeLike