What’s redox? We never learned that!
Yes, you did. I use the term “redox” to refer to all of the following chapters in Heinemann Chemistry 2, which you will have learned at the end of Term 3 (September).
- Chapter 26: Redox (revision of Year 11)
- Chapter 27: Galvanic Cells
- Chapter 28: Electrolytic Cells
Don’t underestimate redox
The VCAA has consistently used redox to discriminate which schools and students have the self-discipline required to keep studying at the end of the year. Studies show that redox is taught at a time when student motivation is at its minimum: energy levels are low, emotions are high, and graduation is just over the horizon. Many schools and students gloss over these topics because they’re running out of time, any many students think they’ve grasped the topic – when they’ve actually grasped misconceptions instead.
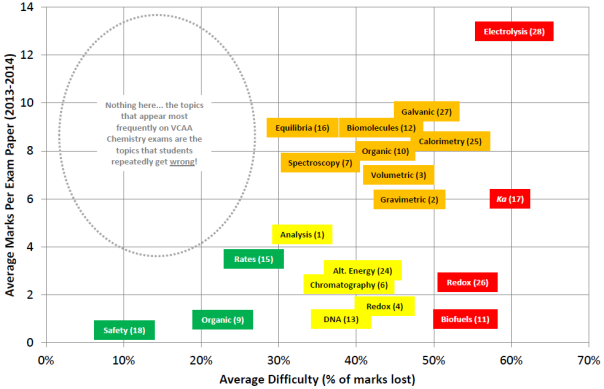
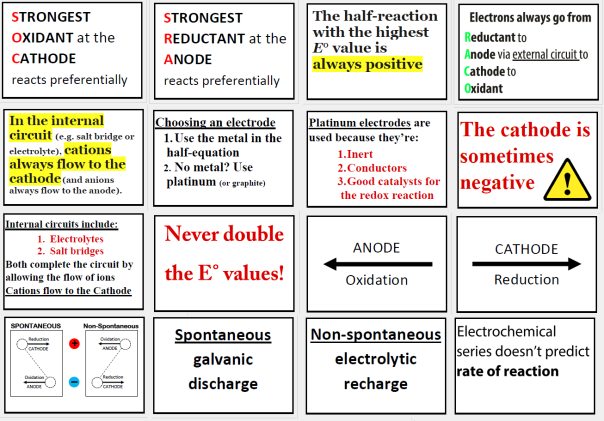
Here are some popular redox lies (misconceptions)
LIE #1: The polarities switch during recharge
Nope. The polarities never switch. It’s the labels of ‘anode’ and ‘cathode’ that switch because the electrons are flowing the other way through the external circuit. Polarity is permanent.
LIE #2: Hydrogen fuel cells don’t emit any greenhouse gases
Wrong. They emit H2O, which is a powerful greenhouse gas. If you don’t believe that the VCAA can be this pedantic, think again. Read their 2015 Examiners Report here.
LIE #3: Each mole of electrons forms 1 mol Ag, 2 mol Cu or 3 mol Al in a cell
Wrong again. If you look at the half-equations, you’ll see that each mole of electrons actually forms 1 mol Ag, 1⁄2 mol Cu or 1⁄3 mol Al. That’s why I teach “1, 1⁄2 and 1⁄3 moles” instead of the typical “1, 2, 3 moles” rule.
LIE #4: Temperature increases the rate of reaction in electroplating
Wrong! Remember that Faraday’s first law states that m ∝ Q. Because Q = I×t, only those two things – current and time – can affect the mass deposited at the cathode.
LIE #5: Electrons always leave the anode and go towards the cathode
Wrong again. Electrons go RACO: to see what that means, download the posters above. This question appears in recent versions of Chemistry Checkpoints. Give it a try.
LIE #6: The cathode is always positive
Ask your teacher.
LIE #7: Ions flow one way in the salt bridge
Nope. Anions always migrate to the anode; and cations always migrate to the cathode.
LIE #8: KOHES always works for balancing half-equations
KOHES only works for cells with acidic electrolytes. For cells with alkaline electrolytes, which sometimes appear in VCAA papers despite not being in the study design (see page 46 here), you’ll need to use KOHES(OH). Here’s KOHES(OH) explained:
- Do KOHES as normal
- Add the same number of OH–(aq) ions to each side of the half-equation to balance out the H+(aq)
- Cancel and simplify. Remember that H+(aq) + OH–(aq) makes H2O(l). Remember also to cancel out any remaining H2O(l).
LIE #9: I can balance an unbalanced redox equation by putting numbers in the equation
Don’t be fooled by this one! The ONLY way to balance an unbalanced redox equation successfully is to do the following:
- Separate it into two half equations
- Balance them using KOHES or KOHES(OH) as appropriate
- Multiply them and recombine
- Cancel and simplify
- Done!
That’s a lot of work but it’s the only way to do it successfully. If you try to ‘cheat’ by just writing numbers (molar coefficients) in front of the reactants and products, you’ll find that the charges don’t add up, and you’ll get zero marks for the question.
LIE #10: I can break up polyatomic ions to make balancing half-equations easier
Nope! You’re only allowed to separate aqueous species in a half equation or an ionic equation. Because the Mn and O are actually bonded together in a polyatomic ion, you’ll need to write this:
- MnO4–(aq) + 8H+(aq) + 5e– → Mn2+(aq) + 4H2O(l) 2/2 marks
Instead of this:
- Mn7+(aq) + 5e– → Mn2+(aq) 0/2 marks
If in doubt, keep it intact and it’ll cancel out by the end if it’s a spectator ion.
LIE #11: The two reactants that are closest together on the electrochemical series react
Not always true. Use SOC SRA instead, which is explained in the posters above. Still struggling? Ask your teacher or tutor for help.
LIE #12: Oxidants are all on the top of the electrochemical series
They’re actually on the left, and all the reductants can be found on the right side of each half equation in the electrochemical series. There is no top/bottom divide on the electrochemical series: only a left/right divide of oxidants/reductants.
Decorate your school/bedroom/hallway
Surround yourselves with truthful redox revision using these 17 free Redox posters. I’ve had these up around the whiteboard for a few weeks now – they’re a constant reminder to students that redox has many ideas that are always true.
One more tip: print and laminate an electrochemical series (available here) so you can annotate it during dozens of practice dozens without wasting paper. Good luck!
James
Nothiing new I’m afraid. When I taught Nuffield A level chemistry in the 1980s, these chapters always near the end of the course, caused problems. I often feel that IONS are a big problem in understanding from day one and word should be used from day one as well. Students said I taught it well but I found it diffiult to understand so I used to go through it a tortuous, perdantic, first principles approach so that I could get the right answer. Not helped by some books writing the half equations the other way round in those days. I wrote a bit about it in http://microchemuk.weebly.com/2-blog-is-this-supposed-to-happen/archives/08-2014.
I have also devised a flexible ion salt bridge which students can make themselves and use in well plates. This means that the microscale apparatus looks more like the diagrams in the book http://microchemuk.weebly.com/7-chemistry-in-small-volumes.html.
Keep up the good work!
Bob
LikeLike