
Boron is a metalloid: an intermediate between the metals and non-metals. It exists in many polymorphs (different crystal lattice structures), some of which exhibit more metallic character than others. Metallic boron is non-toxic, extremely hard and has a very high melting point: only 11 elements have a higher melting point than boron.
British scientist Sir Humphrey Davy described boron thus:
“[Boron is] of the darkest shades of olive. It is opake[sic], very friable, and its powder does not scratch glass. If heated in the atmosphere, it takes fire at a temperature below the boiling point of olive oil, and burns with a red light and with scintillations like charcoal” – Sir Humphrey Davy in 1809
Initial condition
Before we add the 1.00 mol of boron into our reaction vessel, we need to recall what’s already in there from our experiments so far:
- H2(g): 0.70 mol
- He(g): 1.00 mol
- Li(s): 0.40 mol
- LiH(s): 0.60 mol
- Be(s): 1.00 mol
The temperature of our vessel is 99 °C and the pressure of the gaseous phase is 525.5 kPa.
Now, let’s add our 1.00 mol of boron powder.
Which reactions take place?
Boron reacts with hydrogen gas to produce a colourless gas called borane, BH3(g), according to the following equation[1]:

Boron also reacts with lithium in very complex ways. If we heat the vessel up to 350 °C, we’d expect to see the formation of a boron-lithium system with chemical formula B3Li according to this equation[2]:

Notice that now we’ve heated up our vessel to 350 °C to allow this reaction to happen, the lithium at the bottom of the vessel has melted.
Boron reacts with lithium hydride as well, but only at temperatures around 688 °C. With our vessel’s temperature set at 350 °C, we won’t observe this particular reaction in our experiment.[3]
Some allotropes of boron – in particular, the alpha allotrope that was discovered in 1958 – is capable of reacting with beryllium to form BeB12. Because we’re using beryllium powder, which has semi-random symmetry, we won’t see any BeB12 forming in our vessel. Alpha-boron only exists at pressures higher than around 3500 kPa. At our moderate pressure of only 525.5 kPa, powdered (semi-random) boron will prevail and no BeB12 will form.[4]
For simplicity’s sake, let’s assume that the two reactions above take place with equal preference.
Boron powder reacts with hydrogen gas
Let’s do an ice table to find out how much borane we make.

A quick n/ratio calculation shows us that the hydrogen gas is limiting in this reaction:



We can expect all of the hydrogen gas to react with the boron powder.
| units are mol | 2 B | 3 H2 | 2 BH3 |
| I | 0.50 | 0.70 | 0 |
| C | -0.466 | -0.70 | +0.466 |
| E | 0.0333 | 0 | 0.466 |
Borane is very unstable as BH3, and it would probably dimerise into B2H6(g). This is still a gas at 350 °C and is much more stable than BH3. For the rest of this experiment we’ll assume that our 0.466 mol of BH3 has dimerised completely into 0.233 mol of B2H6.
Boron powder reacts with lithium
With the molar ratios present in our vessel, at 350 °C, we’d expect to witness the formation of a boron-lithium system, with chemical formula B3Li.

A quick n/ratio calculation shows that in this reaction, the boron powder is limiting.


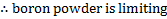
All of the remaining boron therefore reacts with lithium. To calculate exactly how much B3Li we’ve created, let’s do another ice table:
| units are mol | 3 B | Li | B3Li |
| I | 0.533 | 0.40 | 0 |
| C | -0.533 | -0.178 | +0.178 |
| E | 0 | 0.222 | 0.178 |
What’s in our vessel after adding boron?
We have the following gas mixture in our vessel:
Helium gas, He(g): 1.00 mol
Helium is an inert noble gas that will probably remain in the vessel until the end of the experiment. It’s used in party balloons.
Borane gas, B2H6(g): 0.233 mol
We made this today. Borane is used in the synthesis of organic chemicals via a process called hydroboration. An example of hydroboration is shown below.

At the bottom of the vessel, there’s a sludge, which contains the following liquids and solids:
Molten lithium, Li(l): 0.22 mol
Lithium is used in the production of ceramics, batteries, grease, pharmaceuticals and many other applications. We’ve got 0.22 moles of lithium, which is about 1.5 grams.
Beryllium powder, Be(s): 1.00 mol
Beryllium is used as an alloying agent in producing beryllium copper, which is used in springs, electrical contacts, spot-welding electrodes, and non-sparking tools.
Lithium hydride, LiH(s): 0.60 mol
Lithium hydride is used in shielding nuclear reactors and also has the potential to store hydrogen gas in vehicles. Lithium hydride is highly reactive with water.
Boron-lithium system, B3Li(s): 0.178 mol
We made this today… but what is it? Not much is known about this compound – in fact, it doesn’t even have a name other than “boron-lithium system, B3Li”. It’ll probably decompose eventually in our experiment – maybe when we alter the pressure or temperature of the vessel at some later stage. We’ll need to keep an eye on this one.
The original H2(g) and B(s) have been reacted completely in our experiment.
What’s the pressure in our vessel now?
At the end of our reaction, the temperature of our vessel is still set at 350 °C and the pressure of the gaseous phase inside the vessel can be calculated to be a moderate 638 kPa as follows:



*It should also be noted that some evidence exists for a reaction between LiH and BH3, forming Li(BH4). The reaction seems to take place stepwise with increasing temperature. A quick read of this paper suggests that in our vessel, which is at 350 °C, any Li(BH4) formed would actually break back down into boron powder and hydrogen gas, which would in turn react with each other and with lithium metal to form BH3 and LiH again. The net result would be a negligible net gain of LiH and a negligible net loss of boron powder. We will continue calculating this Periodic Table Smoothie under the assumption that if any Li(BH4) forms, it breaks down before we add the next element, and the overall effect on our system is negligible.
**Li(BH4) is an interesting compound: it’s been touted as a potential means of storing hydrogen gas in vehicles – it’s safer and releases hydrogen more readily than LiH, which was mentioned above.[5]
Next week, we’ll add element number 6, carbon, and see what happens.
References
- “Borane”. Wikipedia. N.p., 2016. Web. 14 Apr. 2016.
- Okamoto, H. “The B-Li (Boron-Lithium) System”. Bulletin of Alloy Phase Diagrams 10.3 (1989): 230-232.
- Matkovich, V. I. Boron And Refractory Borides. Berlin: Springer-Verlag, 1977. Print.
- Gaulé, G. K. Boron, Volume 2: Preparation, Properties And Applications. New York: Plenum Press, 1965. Print.
- Saldan, Ivan. “A Prospect For Libh4 As On-Board Hydrogen Storage”. Open Chemistry 9.5 (2011): n. pag. Web.