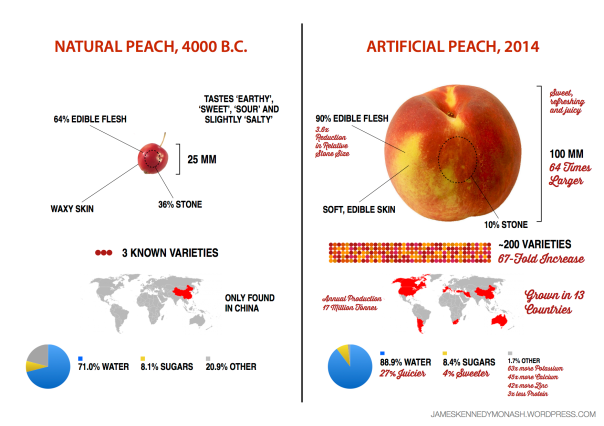
Since 1996, there has existed a niche group of conspiracy theorists in western countries that believes that the government (or some other authority) is spraying compounds out the back of commercial/military aircraft for a plethora of reasons. Seventeen percent of Americans believe a hilariously-named “SLAP” project (secret large-scale atmospheric program) exists in the United States, and 2% are ‘certain’ of its existence. Conspiracy theorists photograph normal aeroplane contrails and upload them to the internet, calling them ‘chemtrails’, and using them as evidence of SLAP.
The conspiracy theorists cite “mind control”, “radar mapping”, and “chemical weapons testing” among suspected motives, and they even have detected elevated concentrations of barium and aluminium in soil and atmosphere at certain locations. Conspiracy theorists use these chemical data to support their belief in the SLAP idea.
Just this month, the results of a comprehensive review of all the so-called evidence for contrails was conducted – by an impressive 77 experts in atmospheric chemistry – and they’ve concluded that the conspiracy theory seems highly unlikely to be true.
First, what are contrails?
Contrails are ice-clouds that emerge from the backs of jet engines on aeroplanes. They vary in width, colour and persistence depending on the temperature, air pressure and humidity.
Combustion in jet engines produces two products: water vapour, H2O(g), and carbon dioxide, CO2(g). These gases exit the jet engine and quickly lose momentum, eventually forming a trail in the air behind the aeroplane. The freezing cold temperatures at aeroplane altitudes freezes the water vapour in its tracks (but not the carbon dioxide – it’s not that cold!). A contrail is essentially a trail of snowflakes!
What did the scientists find?
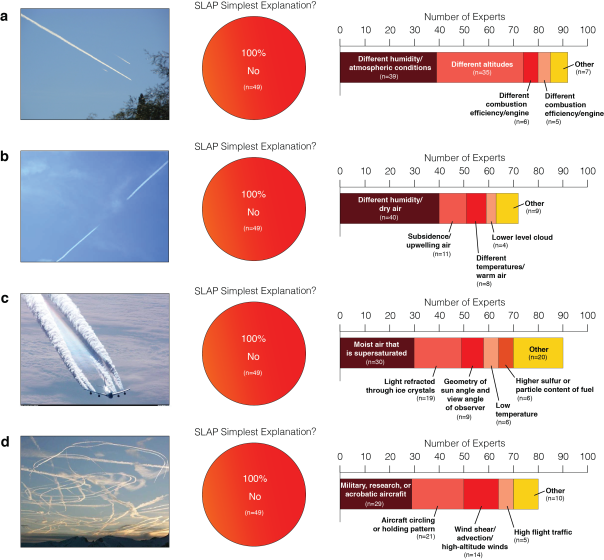
Seventy-seven experts found 100% agreement that SLAP was not the simplest/most likely explanation for the following phenomena:
Why am I mentioning this?
The ‘chemtrails’ conspiracy emerged as one of the most recent forms of chemophobia. It originated in 1996 when a paper was published by the United States Air Force called Weather as a Force Multiplier: Owning the Weather in 2025 suggested spraying compounds from aeroplanes to help engineer the climate. This seeded the conspiracy, and ebbing public trust of experts/scientists helped it to balloon out of proportion from there.
Until this study was conducted, the scientific community had no credible evidence to the contrary: we had no rebuttal to offer the ‘chemtrails’ crowd. This study finally puts the overwhelming majority of evidence (and 76 of the 77 experts involved) in favour of there being no such SLAP project – and no ‘chemtrails’ to speak of.
Chemophobia
It’s widespread, irrational, harmful, and hard to break. One excerpt from a New York Times article on this story said:
“The goal, the researchers say, is not so much to change the minds of hard-core believers, but to provide a rebuttal — the kind that would show up in a Google search — to persuade other people to steer clear of this idea.”
This study, it seems, is aimed at the neutral 60%. This is exactly how we need to be fighting chemophobia.
Question: Have similar studies been conducted for the other forms of chemophobia that exist?